| Drug Name |
3-Sulfinoalanine
|
| Synonyms |
2-amino-3-sulfinopropanoic acid; 2381-08-0; Alanine 3-sulfinic acid; Cysteine hydrogen sulfite ester; L-Cysteinesulfinic acid monohydrate; Alanine, 3-sulfino-; UNII-56X032NVQL; 56X032NVQL; Cysteinesulfinic acid, L-; AC1Q5S9C; MLS000859909; SCHEMBL443654; AC1L18I0; GTPL4695; CHEMBL1702607; 2-Amino-3-sulfinopropionic acid; BDBM86194; NSC_109; CAS_109; ADVPTQAUNPRNPO-UHFFFAOYSA-N; HMS3369H02; HMS3266C09; HMS2235M21; EINECS 214-228-5; AKOS022145786; SMR000326770; ST45022126; 207121-48-0
|
| Drug Type |
Small molecular drug
|
| Structure |
|
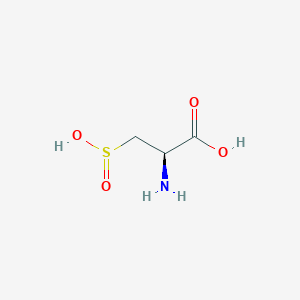 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight (mw) |
153.16 |
|
| Logarithm of the Partition Coefficient (xlogp) |
-4.2 |
| Rotatable Bond Count (rotbonds) |
3 |
| Hydrogen Bond Donor Count (hbonddonor) |
3 |
| Hydrogen Bond Acceptor Count (hbondacc) |
6 |
| Chemical Identifiers |
- Formula
- C3H7NO4S
- IUPAC Name
(2R)-2-amino-3-sulfinopropanoic acid - Canonical SMILES
-
C([C@@H](C(=O)O)N)S(=O)O
- InChI
-
InChI=1S/C3H7NO4S/c4-2(3(5)6)1-9(7)8/h2H,1,4H2,(H,5,6)(H,7,8)/t2-/m0/s1
- InChIKey
-
ADVPTQAUNPRNPO-REOHCLBHSA-N
|
| Cross-matching ID |
- PubChem CID
- 1549098
- ChEBI ID
-
- CAS Number
-
- UNII
-
- DrugBank ID
-
- TTD ID
- D0E8JM
|
|
|
|
|
|
|
|

